Abstract
Chronic graft-vs-host disease (cGVHD) is a major obstacle to the success of allogeneic hematopoietic stem cell transplantation (HCT) in patients. This debilitating condition is characterized by chronic inflammation, cell-mediated and humoral immunity, and ultimately tissue fibrosis. There is currently little or no understanding of the molecular pathogenesis of chronic cGVHD resulting in poor effective treatment strategies. Sclerodermatous GVHD (sclGVHD) is one of the more severe forms of cGVHD associated with poor prognosis and low sensitivity to immune suppressive therapy.
Methods: To address the current gap in knowledge pertaining to the underlying pathophysiology of sclGVHD we used single cell RNA sequencing analyses on fresh patient biopsy specimens. In vivo studies were carried out by sub lethal irradiation of BALB.k recipients which underwent HCT from miAg-mismatched AKR/J donors. Recipient sclerodermatous-tissues were analyzed using FACS, IHC and IF staining. Human studies were conducted on (i) Primary samples from patients with severe sclGVHD using tissue microarrays (TMA) by Immuno-histofluorescence (IHF) and IF. (ii) Dermal fibroblasts from sclGVHD samples were subjected to ATACseq and ChiPseq CRISPR-Cas9 JUN deletion. (iii) Also, dermal fibroblasts from human scl-GVHD were implanted under the kidney capsule of NSG mice to study the effects of inhibiting pro-fibrotic pathways in vivo.
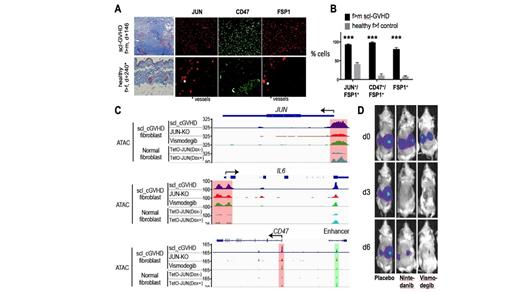
Results: We show for the first time that in a mouse model of sclGVHD (male), recipients of female T-cell replete grafts developed severe scleroderma with massive skin thickening and collagen deposition. Fibroblasts strongly expressed JUN, which is part of AP-1, a transcription factor involved in the acute phase response that regulates gene expression in response to stimuli from cytokines, growth factors and pathogens. We have previously demonstrated JUN as a key player in the molecular pathogenesis of other fibrotic diseases (Wernig G et al. PNAS 2017, Cui et al. Nature comm. 2020, Lerbs et al. JCI i 2020). Likewise, CD47, an immune checkpoint protein that prevents removal of Mϕ, was strongly co-expressed in fibroblasts in sclGVHD - but not in the control mice (Fig A+B). Here we show that (i) In humans, (n = 45 sclGVHD patients), there is a strong expression and activation of JUN and CD47 in dermal fibroblasts which was not observed in control samples. Mixed inflammatory infiltrates were dominated by Mϕ and granulocytes. (ii) Isolated primary fibroblasts from fresh human sclGVHD skin biopsies analyzed for chromatin accessibility across the genome by ATAC-seq showed wide open accessibility to the JUN promoter, IL-6 promoter and CD47 enhancer and promoter indicating that they play a critical role in regulating the pathogenesis of sclGVHD. In contrast, normal fibroblasts displayed only minimal accessibility to the JUN promoter. We further validated our data using CRISPR-Cas9 knock-down studies on sclGVHD fibroblasts and show that the IL-6 promoter, enhancer and promoter of CD47 are regulated by JUN, with JUN deletion resulting in significant decrease in the promoter binding accessibility to IL-6 and CD47 (Fig C). Further, JUN activity appears to regulate key members of the Hh signaling pathway (GLI1, PTCH1 and PTCH2), as their chromatin accessibility was decreased with JUN deletion. These correlative findings were confirmed by JUN ChIP seq, an assay that identifies binding sites of DNA-associated proteins. (iii) To test our findings in vivo we established xenograft models of primary human sclGVHD by implanting cells under the kidney capsule of NSG mice.All treatments (except placebo) resulted in decreased fibrosis (Fig D), presumably by blocking the activation of JUN (pJUN) and its profibrotic downstream pathway members IL-6 and pSTAT3, as assessed by phospho flow. Conclusion: In our studies we demonstrate that in established SclGVHD, combinatorial therapy consisting of anti-CD47 antibody together with IL6 blockade has the highest potential to translate into a therapeutic intervention given its ability to be more effective than currently used antifibrotic and anti-inflammatory agents in clinic. The findings from our study are significant because we show an important mechanism underlying SclGVHD onset, identify a novel genetic signature that can be targeted, describe a new mouse model and a clinical assay that has a high throughput readout and suggest a treatment regimen for patients.
Arai: Magenta Therapeutics: Research Funding. Shizuru: Forty seven Inc: Other: Inventor on a patent licenses by Forty Seven. Forty seven was acquired by Gilead in 2020; Jasper Therapeutics, Inc.: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Chair of scientific advisory board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal